Real Risks of Unregulated Third-Party Servicing
Medical imaging has revolutionized healthcare from the start but especially over the past 30 years. Today, patients and healthcare providers depend on the safe and effective operation of medical imaging devices to diagnose, monitor and guide treatment of cancer and other diseases.
To ensure that patients are receiving safe and accurate scans, the individuals servicing these devices must be highly trained and following “best practices” processes. Accordingly, their employers must be regulated.
Servicing technicians who work for original equipment manufacturers receive extensive training, shadow more experienced technicians, and return for continuing education on new or updated devices – all under the oversight of the Food and Drug Administration (FDA). However, many medical devices are maintained by unregulated third-party servicers who are not required to undergo the same rigorous training program and do not have to register with FDA. This means that some of these highly complicated machines are serviced by businesses that are not required to meet any proper quality and safety Standards. This means their work is done with no oversight.
Without proper oversight, there is an increased risk for serious patient and operator safety and device performance issues. Below are some examples of what happens when medical imaging equipment is improperly serviced by unregulated third parties:
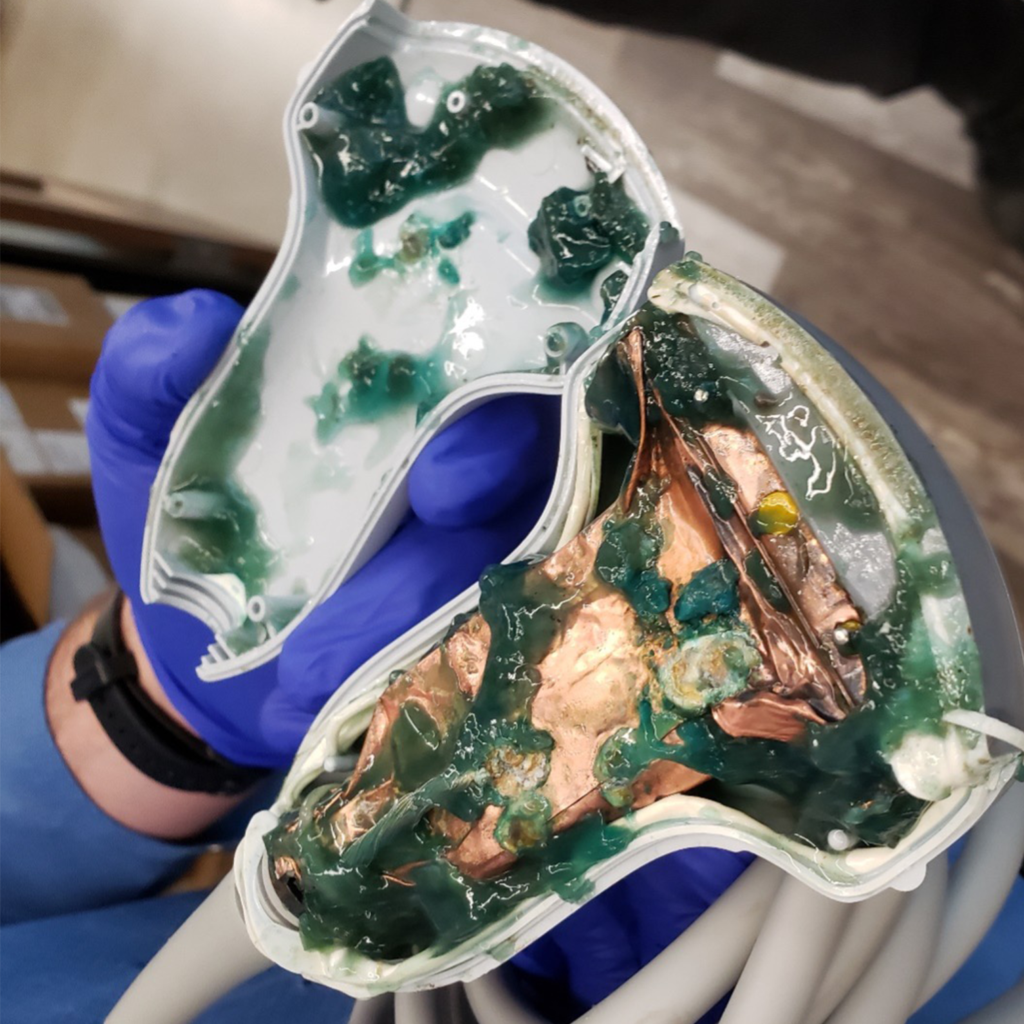
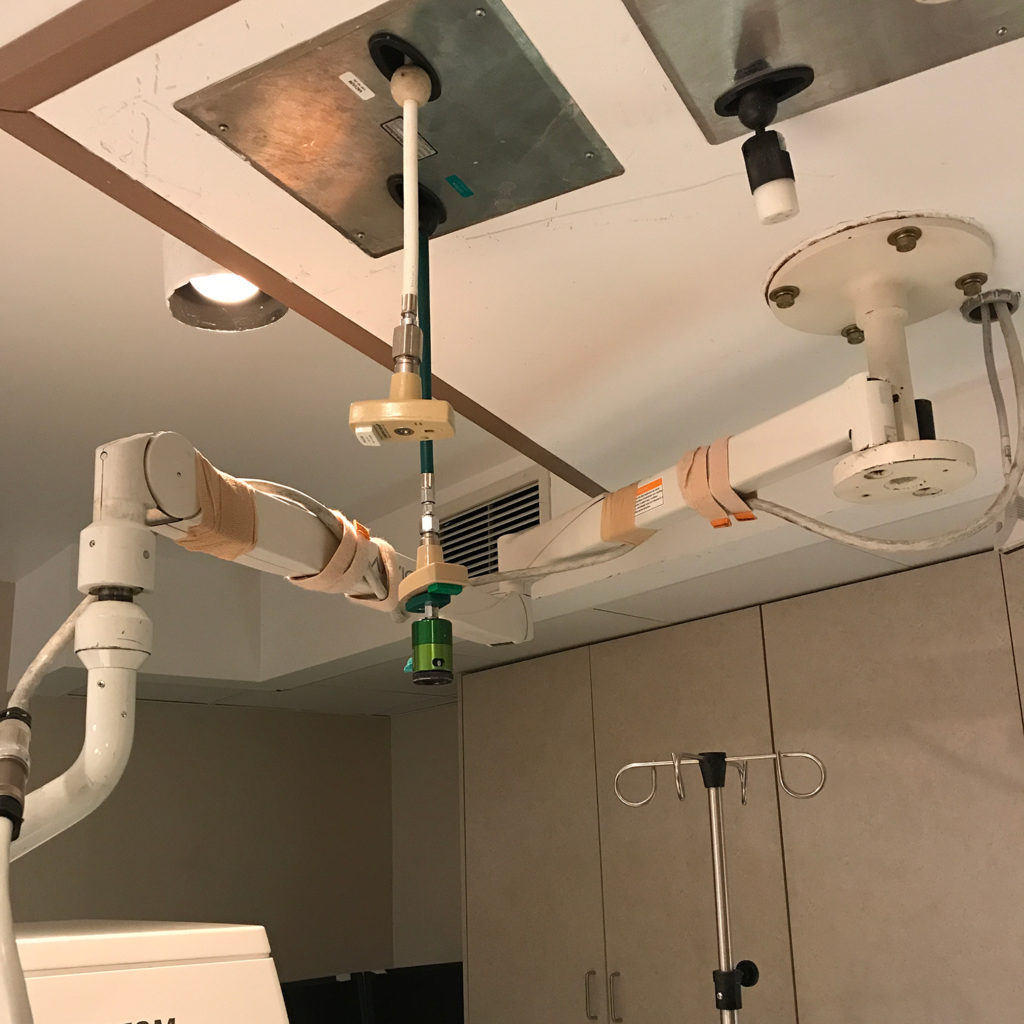


Proper oversight by FDA can help prevent these dangerous situations. FDA should hold all servicing businesses to the same quality, safety, and regulatory requirements, including proper training, implementation of quality and safety controls, adverse event reporting, and registration, to ensure that patients are receiving safe and accurate scans.
More Articles
April is Parkinson’s Disease Awareness Month
April is Parkinson’s Disease Awareness Month! While Parkinson’s has many symptoms, perhaps one of the most well-known indications…
Read MorePatients Deserve Safe Servicing
The medical imaging equipment we rely on to diagnose and monitor cancer and other life-threatening illnesses must be…
Read MoreA Look Ahead to Our Priorities in 2021
Over the last year, America’s healthcare system has been tested like never before. As we continue to grapple…
Read MoreExciting Focused Ultrasound News
Earlier this year, Right Scan Right Time hosted patient leaders in Washington, DC to advocate for expanded access…
Read More